BIOPHAM Mobility
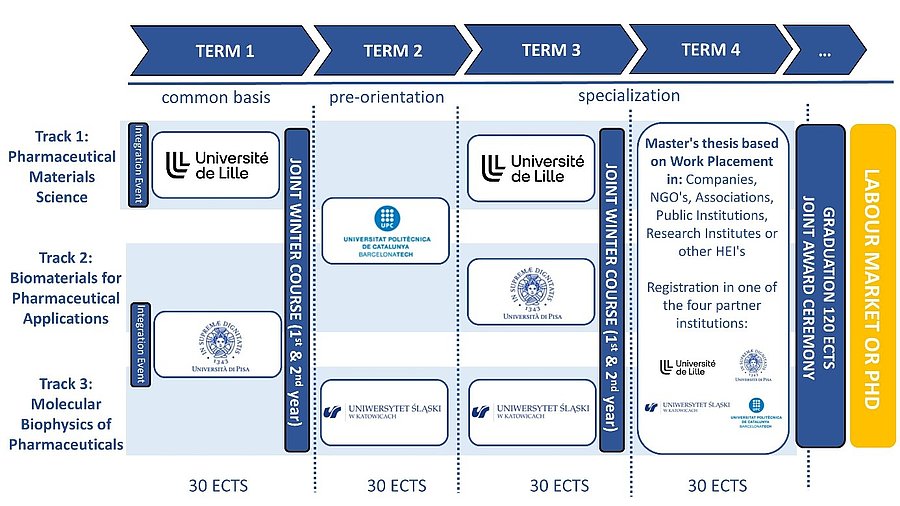
The BIOPHAM Programme is a 2-year study programme. The Master’s diploma is awarded after completion of 120 ECTS: 90 ECTS (3 semesters) consist of courses and practical training, and 30 ECTS of Master’s thesis. The programme courses are taught 100% in English at all partner institutions.
The mobility scheme is as follows :
Integration event
At the beginning of the Master’s programme, students will be welcomed in Lille and in Pisa through dedicated two-day integration events. These gatherings will provide students and teachers with valuable opportunities to meet from the very start, helping to strengthen connections and foster a sense of community. The integration events will include: Greetings and welcome speeches from university authorities and programme directors; Round-table introductions between students and faculty; Testimonies from alumni; sharing their experiences and advice; Q&A sessions to address student questions; Cultural presentations on Pisa and Lille (customs, traditions, student life); Presentations of the programme and courses; Shared lunches and dinners to encourage informal networking and Integration activities such as a treasure hunt or guided visits.These activities are designed not only to introduce students to the academic environment but also to help them integrate smoothly into the social and cultural life of their host cities.
Joint Winter Course (1st and 2nd year students)
Each January, all first-year and second-year students will participate in the joint winter course, co-organized by all partner institutions. These events will be held alternately in Lille and Pisa, taking advantage of the cities where most students reside at that time of year.
The programme is designed to provide students with a comprehensive learning experience on topics not typically taught in the classroom. It will feature:
- Advanced lectures on material sciences, pharmaceuticals, and biomaterials for health applications
- Industry-focused sessions led by professionals on formulation development, manufacturing technologies, and the challenges of industrial scale-up
- Workshops introducing Open Science practices, the FAIR principles (Findable, Accessible, Interoperable, Reusable), as well as topics such as inclusion, diversity, gender equality, dissemination, communication, and public engagement
- Business, strategy, and innovation modules to strengthen career readiness
Beyond the academic content, the winter course is also designed to foster strong interactions among students, professors, invited scholars, and industry experts, who will contribute through lectures and specialized courses.
Thanks to the mobility scheme, this event will bring together both first- and second-year Master’s students, creating valuable opportunities for exchange. In addition, alumni will be invited to attend, further reinforcing programme cohesion and enabling the sharing of insights on mobility, courses, challenges, and aspirations.
Ultimately, this annual event will help students to expand their networks and strengthen their preparation for future careers.
First Term: common basis
During the first term at ULille (track 1) or UNIPI (track 2 and 3), all students will mainly complete a set of core courses covering a broad spectrum of condensed matter physics and materials science topics demonstrating their interests to pharmaceuticals. The primary objective of this first term is to harmonize and strengthen students' foundational knowledge, ensuring they reach the required competency level for their respective tracks.
Second term: pre-orientation
During the second term, all students will relocate to UPC (track 1 and 2) or USK (track 3) depending on the chosen track. This term marks the transition from general coursework to more specialized subjects, allowing students to explore new concepts and approaches through a flexible selection of optional courses. Students will continue to strengthen their fundamental skills. They will also begin specialized training in key areas related to the three tracks. This term provides students with a dynamic and interdisciplinary learning experience, preparing them for further specialization in their respective tracks.
Third-Fourth specialization terms: on the road to employment
The third term will be the specialization term. Students of the Track 1 will go back to Lille. The courses will provide students development skills on the specialized materials science underlying the research pharmaceutical formulation. It will focus on: the different materials used in the field of pharmacy, their physical states, their specific modes of preparation and transformation induced by the typical constraints imposed by pharmaceutical industrial processes. Students in Track 2 will relocate to Pisa, where they will focus on the specialized biomaterials science underlying pharmaceutical research at the crossroad of biomedical and engineering science. It will offer advanced training in the design and construction of materials devices or technologies which interact with living systems, such as medical materials. Students engaged in the Track 3 will move to Katowice to develop their skills on biophysics of molecular materials (polymers, colloids, gels) as well as biological materials (peptides, proteins) of therapeutic interest and their specificities.
The fourth and final term is dedicated to the Master’s thesis. Students have the flexibility to choose their thesis location, which may be: i) a research laboratory at a partner university, ii) an associated academic or industry partner organization or iii) any other company offering a relevant, research-oriented topic for the Master’s thesis. Students will be strongly encouraged to leverage the extensive network of associated academic and industrial organizations, as well as external partner universities. Regardless of the chosen location, a formal agreement will be signed between the student, the host institution (company, laboratory, or organization), and the host university selected for the fourth term among the four partner institutions.
The educational aim of the academic programme is to qualify students to a level of excellence in one of the 3 specialised professionally oriented fields which have been identified:
• Track 1: Pharmaceutical materials science
• Track 2: Biomaterials for pharmaceutical applications
• Track 3: Molecular biophysics of pharmaceuticals
All tracks are offering both “modelling and simulation” and “advanced experimental characterization techniques” related courses.
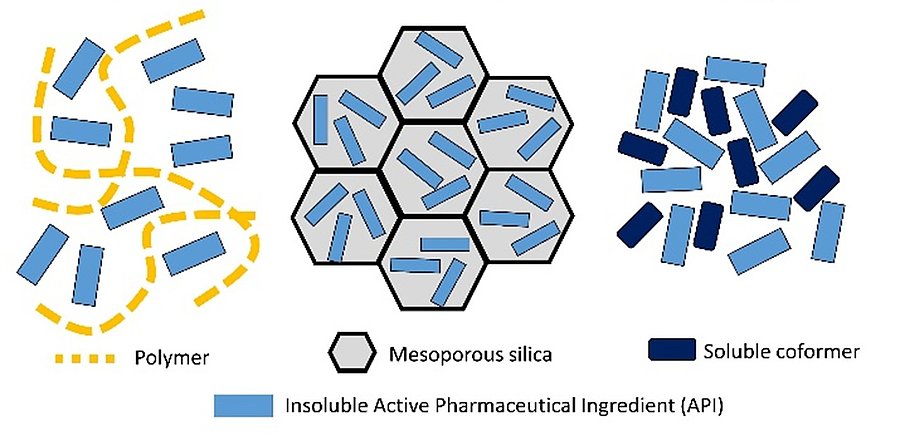
The “Track 1: Pharmaceutical materials science” will offer a specialized training focused on the core of “materials science.” This track emphasizes the study of different physical states (perfect and imperfect crystals, nanocrystals, and glassy states) of small molecules, as well as their surface and interface properties. Effective drugs critically depend on their aqueous solubility and ability to cross biological membranes. It has been noted that a significant proportion, about 70–90%, of new chemical entities (NCEs) in development exhibit poor water solubility, categorising them as either class II or class IV according to the Biopharmaceutics Classification System (BCS). This issue often arises from the molecular structures and functional groups. Consequently, the pharmaceutical industry faces a growing number of NCEs with poor aqueous solubility, challenging the formulation development process. Such NCEs are often categorised into 'brick-dust' molecules (with solubility limitations due to organised crystalline structures) or 'grease-ball' molecules (with solubility issues arising from high lipophilicity). For example, Itraconazole is almost insoluble in water with a solubility of about 1 μg / mL (or less), which is 100 million times lower than the solubility of NaCl salt. Thus, enhancing aqueous solubility and membrane permeability is essential for successfully developing drug formulations. Multiple formulation techniques to enhance the inherent solubility and permeability have been investigated to avoid chemical modifications of the NCEs, and, according to published studies, several methods have proven effective. These include micronisation, crystalline metastable polymorphs, co-crystals and salts, and nanocrystals, to cite only a few. Amorphous pharmaceuticals are an innovative segment in drug development, distinguished by their non-crystalline structure. Unlike traditional drugs that are often crystalline, amorphous substances lack a defined long-range molecular arrangement, leading to distinct properties and advantages. The primary advantage of amorphous drugs lies in their enhanced apparent solubility. Solubility enhancement ratios ranging from about 10 to 1000 are often reported for various drugs such as ibuprofen, indomethacin, and griseofulvin. The absence of a rigid crystalline structure allows these substances to dissolve more readily in biological fluids, potentially increasing their bioavailability. This is especially beneficial for drugs that inherently have low solubility in water, as it can significantly improve their absorption and effectiveness in the body. Moreover, amorphous pharmaceuticals tend to have faster dissolution rates than their crystalline counterparts. This rapid dissolution can translate into quicker therapeutic effects, which is advantageous for medications that require immediate action. For ritonavir, a classic HIV protease inhibitor, the dissolution rate of the amorphous solid can be ~10 times faster than its crystalline form. Despite these benefits, amorphous pharmaceuticals also present clear formulation challenges. They are susceptible to thermodynamic instability (conversion from an amorphous state to a crystalline state) due to the higher free energy associated with the amorphous state. This transformation negates the advantages of using the amorphous state. Their lack of physical stability can lead to issues with long-term storage and consistency of the drug's efficacy. A common approach for the stabilisation of amorphous solids of a drug is the formation of a so-called amorphous solid dispersion (ASD) (see Figure).
Schematic representation of the three types of amorphous formulations which have recently attracted a considerable attention for insoluble Active Pharmaceutical Ingredients (APIs). Left: API dispersed in polymer. Middle: nanoconfined API in mesoporous silica. Right: API/coformer in co-amorphous form. Students in Track 1 will be trained with the right skillset to design, characterise and make full use of these challenging complex systems.
Companies are particularly seeking professionals with a strong background in the solid-state physical characterization of small molecules (e.g., dielectric relaxation, Raman and infrared spectroscopies, NMR, X-ray powder diffraction, DSC, and TGA thermal analyses). They are also looking for expertise in optimizing the performance (e.g., solubility, stability, controlled release) of single-component crystalline and amorphous drug substance samples, as well as in the design of multicomponent materials (e.g., cocrystalline and coamorphous forms) using various techniques such as mechanosynthesis, spray-drying, freeze-drying, hot-melt extrusion and solvent evaporation.
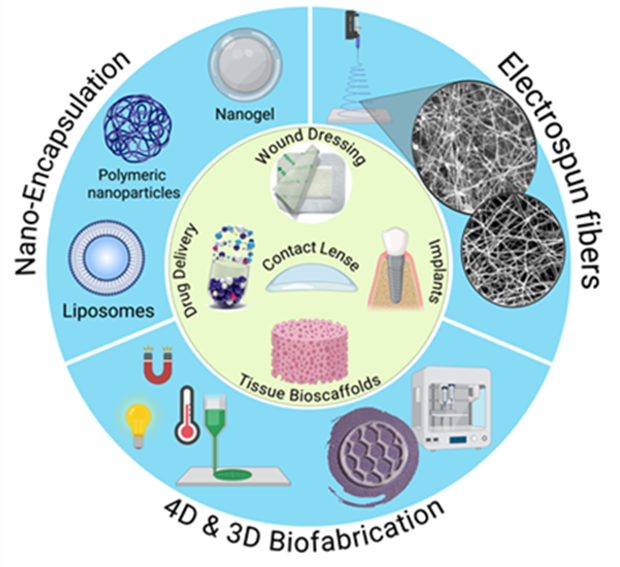
The “Track 2 Biomaterials for Pharmaceutical Applications” will cover the fundamental principles of biomaterials, with a focus on their design, characterization, and applications in healthcare and pharmaceutical sciences (see Figure). In 2023, the Biomaterials market was valued at approximately $178 billion and is projected to grow at a compound annual growth rate (CAGR) of around 15.6% from 2024 to 2030, with a growing share of the market coming from emerging technologies like 3D printing and nanomaterials. As the healthcare landscape continues to evolve, the demand for more sophisticated biomaterials, particularly in areas like regenerative medicine, drug delivery, and personalized treatments, including cancer ones, experts able to deal with such a complex and multidisciplinary scenario are becoming strategic. The students of this track will gain an understanding of the structure-property relationships of biomaterials, including natural, biotech and synthetic origin, their interactions with biological systems, and their role in advancing innovative healthcare solutions, including drug delivery systems on different scales (nano-micro-macro), implants, and tissue engineering scaffolds. Innovative technologies, such as electrospinning, 3D and 4D printing and bioprinting, along with conventional technologies (e.g., emulsions, freeze drying) and hands-on laboratory experience, regulatory and sustainability aspects, will inspire the students to next generation pharmaceutical biomaterials. By the end of this training track, students will be able to: i) know the advanced (bio)fabrication techniques (from macro-to-nanoscale), the modern analytical, structural and imaging techniques for characterization of biomaterials, and the most important regulatory aspects for clinical translation; ii) understand the key properties of biomaterials and their relevance to biomedical applications, including the interactions between biomaterials and biological systems and biocompatibility, iii) explore the applications of biomaterials in drug delivery, regenerative medicine, and pharmaceutical technologies, and iv) develop skills in selecting and designing biomaterials for specific medical and pharmaceutical purposes.
The “Track 3: Molecular biophysics of pharmaceuticals” will focus on training students to understand, at the molecular level, the physical principles that govern the structure, dynamics, and interactions of biomolecules and pharmaceuticals. This knowledge is crucial for designing more effective drugs, improving drug delivery, and developing new therapeutic strategies, providing a foundation for the next generation of researchers and professionals working in drug design, nanomedicine, and biopharmaceuticals. Biopharmaceuticals are among the most sophisticated medicines and are becoming increasingly prominent in the pharmaceutical industry. Some companies are already allocating 40% or more of their R&D budgets to biopharmaceuticals, which are expected to dominate future product usage and sales.
However, compared to conventional chemical drugs, therapeutic proteins face significant challenges due to their intrinsic instability. This has resulted in a strong focus on developing highly stable biopharmaceutical products, particularly solid formulations with enhanced performance. Moreover, companies incur substantial trial-and-error costs in pharmaceutical development, stemming from the absence of a rational approach based on a comprehensive, multi-dimensional understanding of these materials. To address the challenges posed by the physical and chemical instability of protein-based therapies, as well as the limited solubility of synthetic drugs, the demand for targeted delivery, reduced toxicity, and ensuring long-term efficacy, the industry has a high demand for professionals with strong fundamental knowledge in biophysics and material science to address these complex issues effectively (see Figure). This includes expertise in formulating solid forms using advanced drying techniques such as freeze-drying, spray-drying, supercritical fluid drying, and hot melt extrusion techniques. This area requires a solid understanding of materials science, including processes like freezing, thawing, interfacial stress, and interactions with specific solvents (e.g., water and sugars), as well as a thorough understanding of rheological properties. It also encompasses knowledge of stabilization mechanisms (e.g., glassy state formation, water replacement, and interfacial adsorption) and proficiency in specialized experimental techniques such as microcalorimetry, infrared spectroscopy, and Raman scattering.
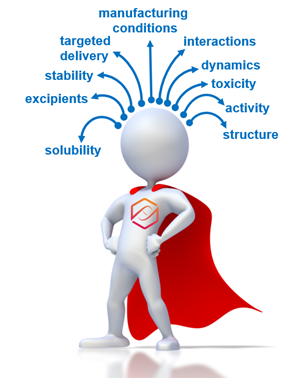
Schematic representation of a BIOPHAM student facing complex drug discovery and development challenges. Track 3 equips students with the biophysical skills and interdisciplinary knowledge required for success. His/her key strength will be a unique, multifaceted (illustrated by arrows) expertise that will allow him/her to anticipate and effectively solve problems relevant to pharmaceutical development by taking into account the molecular and physical conditions of a given material, therapeutic approach, or production process.
In addition to the three tracks described, the programme also includes a specialized training on some advanced experimental and numerical tools for which it exists a clear need for specialized education and training in the field. Students will get the opportunity to specialize on numerical techniques applied to pharmaceutical and biopharmaceutical materials (atomistic modelling, computational biophysics, stochastic methods, artificial intelligence and machine learning with neural networks) and on dedicated advanced characterization experimental techniques including calorimetric techniques (DSC, MDSC), spectroscopic techniques (NMR, Raman, FTIR, UV, Broad Band Dielectrics), X-Ray diffraction and electronic microscopies (SEM/TEM) and also techniques available at synchrotron and neutron source applications (large-scale facilities).